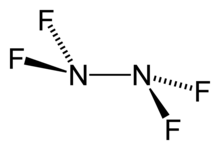
Tetrafluorohydrazine
 | |
 | |
| Names | |
|---|---|
| IUPAC name 1,1,2,2-tetrafluorohydrazine | |
| Other names Tetrafluorohydrazine, perfluorohydrazine, UN 1955 | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider |
|
| ECHA InfoCard | 100.030.091 |
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | N2F4 |
| Molar mass | 104.008 g·mol−1 |
| Appearance | Colourless gas |
| Melting point | −164.5 °C (−264.1 °F; 108.6 K)[1] |
| Boiling point | −73 °C (−99 °F; 200 K)[1] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards | Explosion |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) | 10 mL/kg (rat, intraperitoneal)[2] |
LC50 (median concentration) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).  N verify (what is N verify (what is  Y Y N ?) N ?) Infobox references | |
Tetrafluorohydrazine or perfluorohydrazine, N2F4, is a colourless, nonflammable,[2] reactive inorganic gas. It is a fluorinated analog of hydrazine.
Synthesis
Tetrafluorohydrazine is manufactured from nitrogen trifluoride using an iron catalyst or iron(II) fluoride.[citation needed]
Alternate procedures include the reduction of nitrogen trifluoride with hot arsenic, or oxygen-fluorine exchange between nitrogen trifluoride and nitric oxide.[3]
Properties
Tetrafluorohydrazine is in equilibrium with its radical monomer nitrogen difluoride.[4]
- N2F4 ⇌ 2 •NF2
At room temperature N2F4 is mostly associated with only 0.7% in the form of NF2 at 5mm Hg pressure. When the temperature rises to 225 °C, it mostly dissociates with 99% in the form of NF2.[5]
The energy needed to break the N−N bond in N2F4 is 20.8 kcal/mol, with an entropy change of 38.6 eu.[5] For comparison, the dissociation energy of the N−N bond is 14.6 kcal/mol (61 kJ/mol) in N2O4, 10.2 kcal/mol (43 kJ/mol) in N2O2, and 60 kcal/mol (250 kJ/mol) in N2H4. The enthalpy of formation of N2F4 (ΔfH°) is 34.421 kJ/mol.[6]
Uses
Tetrafluorohydrazine is used in organic synthesis and some rocket propellant formulations.[2] It adds across double bonds to give vicinal di(difluoroamine)s.[7] In chemical syntheses, as a precursor or a catalyst. It was considered for use as a high-energy liquid oxidizer in some never-flown rocket fuel formulas in 1959.[8]
Safety
Tetrafluorohydrazine is a highly hazardous chemical that explodes in the presence of organic materials.[2]
It is a toxic chemical which irritates skin, eyes and lungs. It is a neurotoxin and may cause methemoglobinemia. May be fatal if inhaled or absorbed through skin. Vapors may be irritating and corrosive. It is a strong oxidizing agent. Contact with this chemical may cause burns and severe injury. Fire produces irritating, corrosive and toxic gases. Vapors from liquefied gas are initially heavier than air and spread across the ground.[2]
Tetrafluorohydrazine explodes or ignites on contact with reducing agents at room temperature, including hydrogen, hydrocarbons, alcohols, thiols, amines, ammonia, hydrazines, dicyanogen, nitroalkanes, alkylberylliums, silanes, boranes or powdered metals. Prolonged exposure of the container of tetrafluorohydrazine to high heat may cause it to rupture violently and rocket. Tetrafluorohydrazine itself can explode at high temperatures or with shock or blast when under pressure. When heated to decomposition in air, it emits highly toxic fumes of fluorine and oxides of nitrogen.[2]
There is a fatal case in which during opening of valves to check the pressure, the cylinder exploded, killing one man and injuring another.[2]
References
- ^ a b Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ a b c d e f g h "Tetrafluorohydrazine". pubchem.ncbi.nlm.nih.gov. National Library of Medicine. Retrieved 26 March 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ^ Clark, John Drury (1972). Ignition! (PDF). New Brunswick, NJ: Rutgers UP. p. 82. ISBN 0-8135-0725-1. LCCN 72-185390.
- ^ Jäger, Susanne; von Jouanne, Jörn; Keller-Rudek, Hannelore; Koschel, Dieter; Kuhn, Peter; Merlet, Peter; Rupecht, Sigrid; Vanecek, Hans; Wagner, Joachim (1986). Koschel, Dieter; Kuhn, Peter; Merlet, Peter; Ruprecht, Sigrid; Wagner, Joachim (eds.). F Fluorine: Compounds with Oxygen and Nitrogen. Gmelin Handbook of Inorganic Chemistry. Vol. 4. Berlin: Springer. p. 162. doi:10.1007/978-3-662-06339-2. ISBN 978-3-662-06341-5. Retrieved 29 August 2015.
- ^ a b Bohn, Robert K.; Bauer, Simon Harvey (February 1967). "An electron diffraction study of the structures of NF2 and N2F4". Inorganic Chemistry. 6 (2): 304–309. doi:10.1021/ic50048a024. molecule dimensions and angles
- ^ "Nitrogen difluoride NF2(g)". www.chem.msu.su.
- ^ Clark 1972, pp. 167–168.
- ^ Tetrafluorohydrazine at DTIC.mil archived March 12, 2007
- v
- t
- e
| HF | He | |||||||||||||||||
| LiF | BeF2 | BF BF3 B2F4 | CF4 CxFy | NF3 N2F4 | OF OF2 O2F2 O2F | F− | Ne | |||||||||||
| NaF | MgF2 | AlF AlF3 | SiF4 | P2F4 PF3 PF5 | S2F2 SF2 S2F4 SF4 S2F10 SF6 | ClF ClF3 ClF5 | HArF ArF2 | |||||||||||
| KF | CaF2 | ScF3 | TiF3 TiF4 | VF2 VF3 VF4 VF5 | CrF2 CrF3 CrF4 CrF5 CrF6 | MnF2 MnF3 MnF4 | FeF2 FeF3 | CoF2 CoF3 | NiF2 NiF3 | CuF CuF2 | ZnF2 | GaF3 | GeF4 | AsF3 AsF5 | SeF4 SeF6 | BrF BrF3 BrF5 | KrF2 KrF4 KrF6 | |
| RbF | SrF2 | YF3 | ZrF4 | NbF4 NbF5 | MoF4 MoF5 MoF6 | TcF6 | RuF3 RuF4 RuF5 RuF6 | RhF3 RhF5 RhF6 | PdF2 Pd[PdF6] PdF4 PdF6 | AgF AgF2 AgF3 Ag2F | CdF2 | InF3 | SnF2 SnF4 | SbF3 SbF5 | TeF4 TeF6 | IF IF3 IF5 IF7 | XeF2 XeF4 XeF6 XeF8 | |
| CsF | BaF2 | * | LuF3 | HfF4 | TaF5 | WF4 WF6 | ReF6 ReF7 | OsF4 OsF5 OsF6 OsF 7 OsF8 | IrF3 IrF5 IrF6 | PtF2 Pt[PtF6] PtF4 PtF5 PtF6 | AuF AuF3 Au2F10 AuF5·F2 | HgF2 Hg2F2 HgF4 | TlF TlF3 | PbF2 PbF4 | BiF3 BiF5 | PoF4 PoF6 | At | RnF2 RnF6 |
| Fr | RaF2 | ** | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| ↓ | ||||||||||||||||||
| * | LaF3 | CeF3 CeF4 | PrF3 PrF4 | NdF3 | PmF3 | SmF2 SmF3 | EuF2 EuF3 | GdF3 | TbF3 TbF4 | DyF3 | HoF3 | ErF3 | TmF2 TmF3 | YbF2 YbF3 | ||||
| ** | AcF3 | ThF4 | PaF4 PaF5 | UF3 UF4 UF5 UF6 | NpF3 NpF4 NpF5 NpF6 | PuF3 PuF4 PuF5 PuF6 | AmF3 AmF4 AmF6 | CmF3 | Bk | Cf | Es | Fm | Md | No | ||||
- AgPF6
- KAsF6
- LiAsF6
- NaAsF6
- HPF6
- HSbF6
- NH4PF6
- KPF6
- KSbF6
- LiPF6
- NaPF6
- NaSbF6
- TlPF6
- Cs2AlF5
- K3AlF6
- Na3AlF6
and pseudohalogenides
- BaSiF6
- BaGeF6
- (NH4)2SiF6
- Na2[SiF6]
- K2[SiF6]
- CBrF3
- CBr2F2
- CBr3F
- CClF3
- CCl2F2
- CCl3F
- CF2O
- CF3I
- CHF3
- CH2F2
- CH3F
- C2Cl3F3
- C2H3F
- C6H5F
- C7H5F3
- C15F33N
- C3H5F
- C6H11F
lanthanide, actinide, ammonium
- VOF3
- CrOF4
- CrF2O2
- NH4F
- (NH4)2ZrF6
- CsXeF7
- Li2TiF6
- Li2ZrF6
- K2TiF6
- Rb2TiF6
- Na2TiF6
- Na2ZrF6
- K2NbF7
- K2TaF7
- K2ZrF6
- UO2F2
- FNO
- FNO2
- FNO3
- KHF2
- NaHF2
- NH4HF2
and iodosyl
- F2OS
- F3OP
- PSF3
- IOF3
- IO3F
- IOF5
- IO2F
- IO2F3










