Sutezolid
 | |
| Names | |
|---|---|
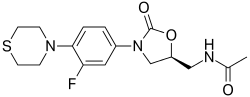
| Preferred IUPAC name N-({(5S)-3-[3-Fluoro-4-(thiomorpholin-4-yl)phenyl]-2-oxo-1,3-oxazolidin-5-yl}methyl)acetamide | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider |
|
| ECHA InfoCard | 100.237.103 |
| KEGG |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | C16H20FN3O3S |
| Molar mass | 353.41 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Infobox references | |
Chemical compound
Sutezolid (PNU-100480, PF-02341272) is an oxazolidinone antibiotic currently in development as a treatment for extensively drug-resistant tuberculosis. It differs from linezolid by replacement of the morpholine oxygen with a sulfur atom.
Notes
- Rapid evaluation in whole blood culture of regimens for XDR-TB containing PNU-100480 (sutezolid), TMC207, PA-824, SQ109, and pyrazinamide
- v
- t
- e
| Aminoglycosides (initiation inhibitors) |
| ||||||
|---|---|---|---|---|---|---|---|
| Tetracycline antibiotics (tRNA binding) |
|
| Steroid antibacterials |
|---|
- #WHO-EM
- ‡Withdrawn from market
- Clinical trials:
- †Phase III
- §Never to phase III
 | This systemic antibiotic-related article is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e











