Methylenetriphenylphosphorane
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name Methylidenetri(phenyl)phosphane | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | C19H17P |
| Appearance | yellow solid |
| Density | 1.19 g/cm3 |
Solubility in water | decompose |
| Solubility | THF |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Infobox references | |
Methylenetriphenylphosphorane is an organophosphorus compound with the formula Ph3PCH2. It is the parent member of the phosphorus ylides, popularly known as Wittig reagents. It is a highly polar, highly basic species.
Preparation and use
Methylenetriphenylphosphorane is prepared from methyltriphenylphosphonium bromide by its deprotonation using a strong base like butyllithium:[1]
- Ph3PCH3Br + BuLi → Ph3PCH2 + LiBr + BuH
The phosphorane is generally not isolated, instead it is used in situ. The estimated pKa of this carbon acid is near 15.[2] Potassium tert-butoxide has been used in place of butyl lithium.[3] Sodium amide has also been used a base.[4]
Methylenetriphenylphosphorane is used to replace oxygen centres in aldehydes and ketones with a methylene group, i.e., a methylenation:
- R2CO + Ph3PCH2 → R2C=CH2 + Ph3PO
The phosphorus-containing product is triphenylphosphine oxide.
Structure
Crystallographic characterization of the colourless ylide reveals that the phosphorus atom is approximately tetrahedral. The PCH2 centre is planar and the P=CH2 distance is 1.661 Å, which is much shorter than the P-Ph distances (1.823 Å).[5] The compound is usually described as a combination of two resonance structures:
- Ph3P+CH2− ↔ Ph3P=CH2
Uses
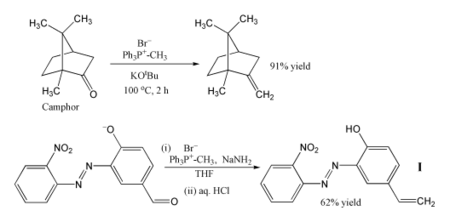
Methylenetriphenylphosphorane has become a standard tool for synthetic organic chemists.[6]
Related reagents
- (Chloromethylene)triphenylphosphorane
- Methoxymethylenetriphenylphosphine
- Carbomethoxymethylenetriphenylphosphorane
References
- ^ Wittig, Georg; Schoellkopf, U. (1960). "Methylenecyclohexane". Organic Syntheses. 40: 66. doi:10.15227/orgsyn.040.0066.
- ^ Ling-Chung, Sim; Sales, Keith D.; Utley, James H. P. (1990). "Measurement of pKa Values for Phosphonium Salts via the Kinetics of Proton Transfer to an Electrogenerated Base". Journal of the Chemical Society, Chemical Communications (9): 662. doi:10.1039/C39900000662.
- ^ Fitjer, L.; Quabeck, U. Synthetic Communications 1985, 15(10), 855–864.
- ^ F. A. Bottino, G. Di Pasquale, A. Pollicino, A. Recca and D. T. Clark (1990). "Synthesis of 2-(2-hydroxyphenyl)-2H-benzotriazole monomers and studies of the surface photostabilization of the related copolymers". Macromolecules. 23 (10): 2662–2666. Bibcode:1990MaMol..23.2662B. doi:10.1021/ma00212a011.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bart, J. C. J. (1969). "Structure of the non-stabilized phosphonium ylid methylenetriphenylphosphorane". Journal of the Chemical Society B. 1969: 350–365. doi:10.1039/J29690000350.
- ^ B. E. Maryanoff & A. B. Reitz (1989). "The Wittig olefination reaction and modifications involving phosphoryl-stabilized carbanions. Stereochemistry, mechanism, and selected synthetic aspects". Chem. Rev. 89 (4): 863–927. doi:10.1021/cr00094a007.













