Protein-coding gene in the species Homo sapiens
| IMPA1 |
|---|
 |
| Available structures |
|---|
| PDB | Ortholog search: PDBe RCSB |
|---|
| List of PDB id codes |
|---|
1AWB, 1IMA, 1IMB, 1IMC, 1IMD, 1IME, 1IMF, 2HHM, 4AS4 |
|
|
| Identifiers |
|---|
| Aliases | IMPA1, IMP, IMPA, inositol monophosphatase 1, MRT59 |
|---|
| External IDs | OMIM: 602064 MGI: 1933158 HomoloGene: 4043 GeneCards: IMPA1 |
|---|
| EC number | 3.1.3.94 |
|---|
| Gene location (Human) |
|---|
 | | Chr. | Chromosome 8 (human)[1] |
|---|
| | Band | 8q21.13 | Start | 81,656,914 bp[1] |
|---|
| End | 81,686,331 bp[1] |
|---|
|
| Gene location (Mouse) |
|---|
 | | Chr. | Chromosome 3 (mouse)[2] |
|---|
| | Band | 3|3 A1 | Start | 10,377,016 bp[2] |
|---|
| End | 10,396,499 bp[2] |
|---|
|
| RNA expression pattern |
|---|
| Bgee | | Human | Mouse (ortholog) |
|---|
| Top expressed in | - secondary oocyte
- jejunal mucosa
- endothelial cell
- tibia
- right ventricle
- Brodmann area 23
- bronchial epithelial cell
- retinal pigment epithelium
- middle temporal gyrus
- rectum
|
| | Top expressed in | - motor neuron
- ciliary body
- retinal pigment epithelium
- iris
- Paneth cell
- facial motor nucleus
- conjunctival fornix
- external carotid artery
- primitive streak
- epithelium of stomach
|
| | More reference expression data |
|
|---|
| BioGPS | 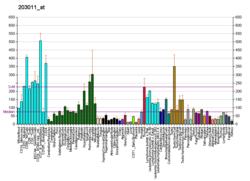 | | More reference expression data |
|
|---|
|
| Gene ontology |
|---|
| Molecular function | - inositol monophosphate phosphatase activity
- manganese ion binding
- protein homodimerization activity
- metal ion binding
- lithium ion binding
- protein binding
- identical protein binding
- hydrolase activity
- magnesium ion binding
- inositol monophosphate 1-phosphatase activity
- inositol monophosphate 4-phosphatase activity
- inositol monophosphate 3-phosphatase activity
| | Cellular component | | | Biological process | - inositol metabolic process
- inositol biosynthetic process
- inositol phosphate metabolic process
- phosphatidylinositol phosphate biosynthetic process
- phosphate-containing compound metabolic process
- phosphatidylinositol biosynthetic process
- signal transduction
- inositol phosphate dephosphorylation
| | Sources:Amigo / QuickGO |
|
| Orthologs |
|---|
| Species | Human | Mouse |
|---|
| Entrez | | |
|---|
| Ensembl | | |
|---|
| UniProt | | |
|---|
| RefSeq (mRNA) | |
|---|
NM_005536
NM_001144878
NM_001144879 |
| |
|---|
| RefSeq (protein) | |
|---|
NP_001138350
NP_001138351
NP_005527 |
| |
|---|
| Location (UCSC) | Chr 8: 81.66 – 81.69 Mb | Chr 3: 10.38 – 10.4 Mb |
|---|
| PubMed search | [3] | [4] |
|---|
|
| Wikidata |
| View/Edit Human | View/Edit Mouse |
|
Inositol monophosphatase 1 is an enzyme that in humans is encoded by the IMPA1 gene.[5][6]
Interacting partners
IMPA1 has been shown to interact with Bergmann glial S100B[7] and calbindin.[8][9]
Chemical inhibitors
L-690,330 is a competitive inhibitor of IMPase activity with very good activity in vitro however with limited bioavailability in vivo.[10] Due to its increased specificity compared to Lithium, L-690,330 has been used extensively in characterizing the results of IMPase inhibition in various cell culture models. L-690,488, a prodrug or L-690,330, has also been developed which has greater cell permeability. Treatment of cortical slices with L-690,488 resulted in accumulation of inositol demonstrating the activity of this inhibitor in tissue.[11]
Inhibition of IMPA1 activity can have pleiotropic effects on cellular function, including altering phosphoinositide signalling,[12] autophagy, apoptosis,[13] and other effects.
Bipolar disorder
Initially it was noticed that several drugs useful in treatment of bipolar disorder such as lithium, carbamazepine and valproic acid had a common mechanism of action on enzymes in the phosphatidylinositol signalling pathway[14] and the inositol depletion hypothesis for the pathophysiology of bipolar disorder was suggested. Intensive research has so far not confirmed this hypothesis, partly because lithium can also act on a number of other enzymes in this pathway, complicating results from in vitro studies.
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000133731 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000027531 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ McAllister G, Whiting P, Hammond EA, Knowles MR, Atack JR, Bailey FJ, Maigetter R, Ragan CI (Aug 1992). "cDNA cloning of human and rat brain myo-inositol monophosphatase. Expression and characterization of the human recombinant enzyme". Biochem J. 284 (3): 749–54. doi:10.1042/bj2840749. PMC 1132602. PMID 1377913.
- ^ "Entrez Gene: IMPA1 inositol(myo)-1(or 4)-monophosphatase 1".
- ^ Vig PJ, Shao Q, Subramony SH, Lopez ME, Safaya E (September 2009). "Bergmann glial S100B activates myo-inositol monophosphatase 1 and Co-localizes to purkinje cell vacuoles in SCA1 transgenic mice". Cerebellum. 8 (3): 231–44. doi:10.1007/s12311-009-0125-5. PMC 3351107. PMID 19593677.
- ^ Schmidt H, Schwaller B, Eilers J (April 2005). "Calbindin D28k targets myo-inositol monophosphatase in spines and dendrites of cerebellar Purkinje neurons". Proc. Natl. Acad. Sci. U.S.A. 102 (16): 5850–5. Bibcode:2005PNAS..102.5850S. doi:10.1073/pnas.0407855102. PMC 556286. PMID 15809430.
- ^ Berggard T, Szczepankiewicz O, Thulin E, Linse S (November 2002). "Myo-inositol monophosphatase is an activated target of calbindin D28k". J. Biol. Chem. 277 (44): 41954–9. doi:10.1074/jbc.M203492200. PMID 12176979.
- ^ Atack JR, Cook SM, Watt AP, Fletcher SR, Ragan CI (February 1993). "In vitro and in vivo inhibition of inositol monophosphatase by the bisphosphonate L-690,330". J. Neurochem. 60 (2): 652–8. doi:10.1111/j.1471-4159.1993.tb03197.x. PMID 8380439. S2CID 23498954.
- ^ Atack JR, Prior AM, Fletcher SR, Quirk K, McKernan R, Ragan CI (July 1994). "Effects of L-690,488, a prodrug of the bisphosphonate inositol monophosphatase inhibitor L-690,330, on phosphatidylinositol cycle markers". J. Pharmacol. Exp. Ther. 270 (1): 70–6. PMID 8035344.
- ^ King JS, Teo R, Ryves J, Reddy JV, Peters O, Orabi B, Hoeller O, Williams RS, Harwood AJ (2009). "The mood stabiliser lithium suppresses PIP3 signalling in Dictyostelium and human cells". Dis Models Mech. 2 (5–6): 306–12. doi:10.1242/dmm.001271. PMC 2675811. PMID 19383941.
- ^ Sarkar S, Rubinsztein DC (2006). "Inositol and IP3 levels regulate autophagy: biology and therapeutic speculations". Autophagy. 2 (2): 132–4. doi:10.4161/auto.2387. PMID 16874097.
- ^ Williams RS, Cheng L, Mudge AW, Harwood AJ (May 2002). "A common mechanism of action for three mood-stabilizing drugs". Nature. 417 (6886): 292–5. Bibcode:2002Natur.417..292W. doi:10.1038/417292a. PMID 12015604. S2CID 4302048.
Further reading
- Bone R, Springer JP, Atack JR (1992). "Structure of inositol monophosphatase, the putative target of lithium therapy". Proc. Natl. Acad. Sci. U.S.A. 89 (21): 10031–10035. Bibcode:1992PNAS...8910031B. doi:10.1073/pnas.89.21.10031. PMC 50271. PMID 1332026.
- Hallcher LM, Sherman WR (1981). "The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain". J. Biol. Chem. 255 (22): 10896–901. doi:10.1016/S0021-9258(19)70391-3. PMID 6253491.
- Bone R, Frank L, Springer JP, Pollack SJ, Osborne S, Atack JR, Knowles MR, McAllister G, Ragan CI (1994). "Structural analysis of inositol monophosphatase complexes with substrates". Biochemistry. 33 (32): 9460–9467. doi:10.1021/bi00198a011. PMID 8068620.
- Bone R, Frank L, Springer JP, Atack JR (1994). "Structural studies of metal binding by inositol monophosphatase: evidence for two-metal ion catalysis". Biochemistry. 33 (32): 9468–9476. doi:10.1021/bi00198a012. PMID 8068621.
- Ganzhorn AJ, Lepage P, Pelton PD, Strasser F, Vincendon P, Rondeau JM (1996). "The contribution of lysine-36 to catalysis by human myo-inositol monophosphatase". Biochemistry. 35 (33): 10957–10966. doi:10.1021/bi9603837. PMID 8718889.
- Parthasarathy L, Parthasarathy R, Vadnal R (1997). "Molecular characterization of coding and untranslated regions of rat cortex lithium-sensitive myo-inositol monophosphatase cDNA". Gene. 191 (1): 81–87. doi:10.1016/S0378-1119(97)00045-0. PMID 9210592.
- Sjøholt G, Molven A, Løvlie R, Wilcox A, Sikela JM, Steen VM (1997). "Genomic structure and chromosomal localization of a human myo-inositol monophosphatase gene (IMPA)". Genomics. 45 (1): 113–122. doi:10.1006/geno.1997.4862. PMID 9339367.
- Nemanov L, Ebstein RP, Belmaker RH, Osher Y, Agam G (1999). "Effect of bipolar disorder on lymphocyte inositol monophosphatase mRNA levels". The International Journal of Neuropsychopharmacology. 2 (1): 25–29. doi:10.1017/S1461145799001315. PMID 11281967. S2CID 16964945.
- Bahn JH, Kim AY, Jang SH, Lee BR, Ahn JY, Joo HM, Kan TC, Won MH, Kwon HY (2002). "Production of monoclonal antibodies and immunohistochemical studies of brain myo-inositol monophosphate phosphatase". Mol. Cells. 13 (1): 21–7. doi:10.1016/S1016-8478(23)14999-5. PMID 11911470.
- Atack JR, Schapiro MB (2002). "Inositol monophosphatase activity in normal, Down syndrome and dementia of the Alzheimer type CSF". Neurobiol. Aging. 23 (3): 389–396. doi:10.1016/S0197-4580(01)00335-9. PMID 11959401. S2CID 24701473.
- Berggard T, Szczepankiewicz O, Thulin E, Linse S (2003). "Myo-inositol monophosphatase is an activated target of calbindin D28k". J. Biol. Chem. 277 (44): 41954–41959. doi:10.1074/jbc.M203492200. PMID 12176979.
- Strausberg RL, Feingold EA, Grouse LH, Derge JG, Klausner RD, Collins FS, Wagner L, Shenmen CM, Schuler GD (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–16903. Bibcode:2002PNAS...9916899M. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Sjøholt G, Ebstein RP, Lie RT, Berle JØ, Mallet J, Deleuze JF, Levinson DF, Laurent C, Mujahed M (2005). "Examination of IMPA1 and IMPA2 genes in manic-depressive patients: association between IMPA2 promoter polymorphisms and bipolar disorder". Mol. Psychiatry. 9 (6): 621–629. doi:10.1038/sj.mp.4001460. PMID 14699425. S2CID 28747842.
- Gerhard DS, Wagner L, Feingold EA, Shenmen CM, Grouse LH, Schuler G, Klein SL, Old S, Rasooly R (2004). "The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–2127. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334.
- Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, Li N, Berriz GF, Gibbons FD, Dreze M (2005). "Towards a proteome-scale map of the human protein-protein interaction network". Nature. 437 (7062): 1173–1178. Bibcode:2005Natur.437.1173R. doi:10.1038/nature04209. PMID 16189514. S2CID 4427026.
- Ohnishi T, Ohba H, Seo KC, Im J, Sato Y, Iwayama Y, Furuichi T, Chung SK, Yoshikawa T (2007). "Spatial expression patterns and biochemical properties distinguish a second myo-inositol monophosphatase IMPA2 from IMPA1". J. Biol. Chem. 282 (1): 637–646. doi:10.1074/jbc.M604474200. PMID 17068342.
External links
- PDBe-KB provides an overview of all the structure information available in the PDB for Human Inositol monophosphatase 1
PDB gallery
-
1awb: HUMAN MYO-INOSITOL MONOPHOSPHATASE IN COMPLEX WITH D-INOSITOL-1-PHOSPHATE AND CALCIUM -
1ima: STRUCTURAL ANALYSIS OF INOSITOL MONOPHOSPHATASE COMPLEXES WITH SUBSTRATES -
1imb: STRUCTURAL ANALYSIS OF INOSITOL MONOPHOSPHATASE COMPLEXES WITH SUBSTRATES -
1imc: STRUCTURAL STUDIES OF METAL BINDING BY INOSITOL MONOPHOSPHATASE: EVIDENCE FOR TWO-METAL ION CATALYSIS -
1imd: STRUCTURAL STUDIES OF METAL BINDING BY INOSITOL MONOPHOSPHATASE: EVIDENCE FOR TWO-METAL ION CATALYSIS -
1ime: STRUCTURAL STUDIES OF METAL BINDING BY INOSITOL MONOPHOSPHATASE: EVIDENCE FOR TWO-METAL ION CATALYSIS -
1imf: STRUCTURAL STUDIES OF METAL BINDING BY INOSITOL MONOPHOSPHATASE: EVIDENCE FOR TWO-METAL ION CATALYSIS -
2hhm: STRUCTURE OF INOSITOL MONOPHOSPHATASE, THE PUTATIVE TARGET OF LITHIUM THERAPY |
|
|---|
| Activity | |
|---|
| Regulation | |
|---|
| Classification | |
|---|
| Kinetics | |
|---|
| Types | |
|---|
Portal: Biology
Biology

 1awb: HUMAN MYO-INOSITOL MONOPHOSPHATASE IN COMPLEX WITH D-INOSITOL-1-PHOSPHATE AND CALCIUM
1awb: HUMAN MYO-INOSITOL MONOPHOSPHATASE IN COMPLEX WITH D-INOSITOL-1-PHOSPHATE AND CALCIUM 1ima: STRUCTURAL ANALYSIS OF INOSITOL MONOPHOSPHATASE COMPLEXES WITH SUBSTRATES
1ima: STRUCTURAL ANALYSIS OF INOSITOL MONOPHOSPHATASE COMPLEXES WITH SUBSTRATES 1imb: STRUCTURAL ANALYSIS OF INOSITOL MONOPHOSPHATASE COMPLEXES WITH SUBSTRATES
1imb: STRUCTURAL ANALYSIS OF INOSITOL MONOPHOSPHATASE COMPLEXES WITH SUBSTRATES 1imc: STRUCTURAL STUDIES OF METAL BINDING BY INOSITOL MONOPHOSPHATASE: EVIDENCE FOR TWO-METAL ION CATALYSIS
1imc: STRUCTURAL STUDIES OF METAL BINDING BY INOSITOL MONOPHOSPHATASE: EVIDENCE FOR TWO-METAL ION CATALYSIS 1imd: STRUCTURAL STUDIES OF METAL BINDING BY INOSITOL MONOPHOSPHATASE: EVIDENCE FOR TWO-METAL ION CATALYSIS
1imd: STRUCTURAL STUDIES OF METAL BINDING BY INOSITOL MONOPHOSPHATASE: EVIDENCE FOR TWO-METAL ION CATALYSIS 1ime: STRUCTURAL STUDIES OF METAL BINDING BY INOSITOL MONOPHOSPHATASE: EVIDENCE FOR TWO-METAL ION CATALYSIS
1ime: STRUCTURAL STUDIES OF METAL BINDING BY INOSITOL MONOPHOSPHATASE: EVIDENCE FOR TWO-METAL ION CATALYSIS 1imf: STRUCTURAL STUDIES OF METAL BINDING BY INOSITOL MONOPHOSPHATASE: EVIDENCE FOR TWO-METAL ION CATALYSIS
1imf: STRUCTURAL STUDIES OF METAL BINDING BY INOSITOL MONOPHOSPHATASE: EVIDENCE FOR TWO-METAL ION CATALYSIS 2hhm: STRUCTURE OF INOSITOL MONOPHOSPHATASE, THE PUTATIVE TARGET OF LITHIUM THERAPY
2hhm: STRUCTURE OF INOSITOL MONOPHOSPHATASE, THE PUTATIVE TARGET OF LITHIUM THERAPY


























